Energy metabolism and glutamate-glutamine cycle in the brain: a stoichiometric modeling perspective
Times cited: 4
Massucci, FA, DiNuzzo, M, Giove, F, Maraviglia, B, Pérez Castillo, I, Marinari, E, De Martino, A.
.
BMC Systems Biology
7
,
103
(2013).
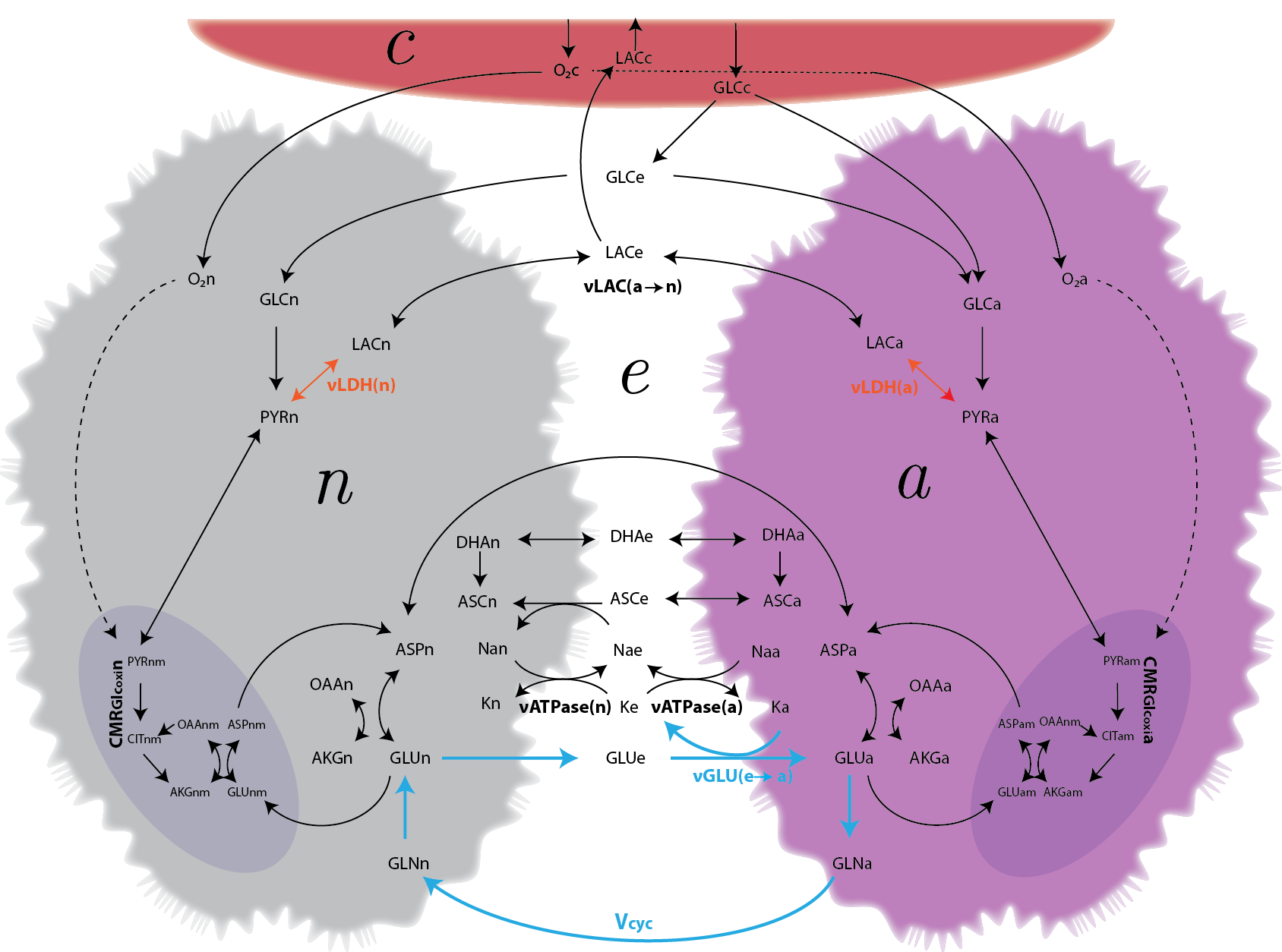
Background: The energetics of cerebral activity critically relies on the functional and metabolic interactions between neurons and astrocytes. Important open questions include the relation between neuronal versus astrocytic energy demand, glucose uptake and intercellular lactate transfer, as well as their dependence on the level of activity.
Results: We have developed a large-scale, constraint-based network model of the metabolic partnership between astrocytes and glutamatergic neurons that allows for a quantitative appraisal of the extent to which stoichiometry alone drives the energetics of the system. We find that the velocity of the glutamate-glutamine cycle (V-cyc) explains part of the uncoupling between glucose and oxygen utilization at increasing V-cyc levels. Thus, we are able to characterize different activation states in terms of the tissue oxygen-glucose index (OGI). Calculations show that glucose is taken up and metabolized according to cellular energy requirements, and that partitioning of the sugar between different cell types is not significantly affected by V-cyc. Furthermore, both the direction and magnitude of the lactate shuttle between neurons and astrocytes turn out to depend on the relative cell glucose uptake while being roughly independent of V-cyc.
Conclusions: These findings suggest that, in absence of ad hoc activity-related constraints on neuronal and astrocytic metabolism, the glutamate-glutamine cycle does not control the relative energy demand of neurons and astrocytes, and hence their glucose uptake and lactate exchange.